Throughout the world, hundreds of millions of people suffer from the disease known as schistosomiasis, which is caused by trematode worms of the genus Schistosoma. Out of the three most medically important species, S. hematobium is the most common species with a presence in 54 countries, particularly in Africa. S. mansoni is endemic in sub-Saharan Africa, Brazil, the Caribbean islands, Puerto Rico, Suriname, and Venezuela, whilst S. japonicum is endemic in parts of the People’s Republic of China and the Philippines. The WHO estimated that 236.6 million people required treatment for schistosomiasis in 2019.
The eggs of the parasite induce an inflammatory response that then lead to several side effects including abdominal pain and diarrhoea (urogenital schistosomiasis), tissue fibrosis and portal vein hypertension or occlusion (intestinal schistosomiasis). Overall, children and adolescents are infected mostly, and, if left untreated, this painful and debilitating disease impairs educational performance and undermines social and economic development. Of note, female genital schistosomiasis has been linked to an increased risk of HIV infections and is now a major focus of World Health Organization (WHO) awareness campaigns.
The current strategy to treat and control schistosomiasis focuses on decreasing the rate of the disease through periodic treatment with the drug, praziquantel (PZQ). This drug causes rapid paralysis of the adult parasite and damage to its surface. Despite the fact that no strong resistance to PZQ has yet to be reported, enormous concern remains regarding the reliance on just one drug to treat whole populations of people. Furthermore, the drug has a number of pharmaceutical and pharmacological drawbacks that encourage the search for new drugs.
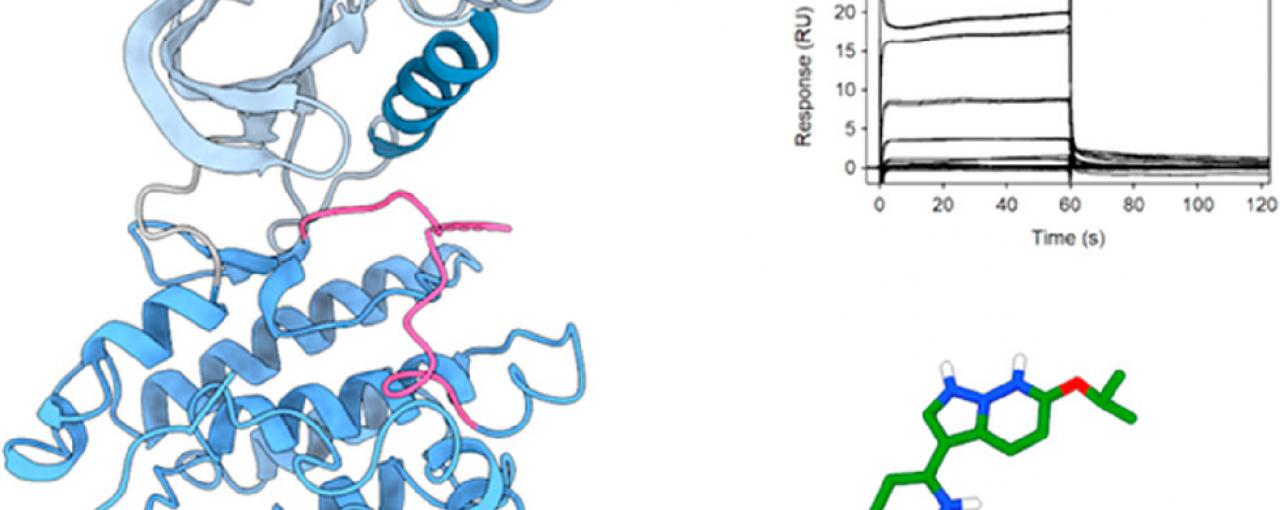
In recent years, the discovery of Venus Kinase Receptors (VKRs) in S. mansoni has offered new directions for schistosomiasis research drug discovery. VKR proteins are composed of a unique extracellular domain that adopt a Venus Flytrap Module (VFTM) and an intracellular kinase domain. VKRs are unique to invertebrates, making them attractive candidates for small molecule inhibition. To date, no VKR structure has been determined. The kinase domain of VKRs share 41% sequence identity with that of insulin receptors (IRs). A known IR inhibitor, tyrphostin AG1024, when applied to the strains SmVKR1 and SmVKR2 caused cells to be destroyed, known as concentration-dependent apoptosis and stopped egg production entirely.
Here at the Research Complex at Harwell, a team led by Dr Konstantinos Beis, Group Leader and Reader in Membrane Protein Structural Biology at Imperial College London, focused on small molecule discovery against the SmVKR2 kinase domain and was able to decipher its crystal structure. The team initiated a drug discovery effort to obstruct the activity of the SmVKR2 kinase domain by using the GlaxoSmithKline published kinase inhibitor set 2 (PKIS2). They identified several inhibitors, four of which were able to also block the activity of SmVKR2 within the parasite which could pave the way to more potent inhibitors against the disease.
The crystal structure of the SmVKR2 displays an active-like state that sheds light on the activation process of VKRs and this can now aid drug discovery efforts by using in silico methods to create more treatments to fight schistosomiasis.
Identification of Inhibitors of the Schistosoma mansoni, Indran Mathavan, Lawrence J. Liu, Sean W. Robinson, Nelly El-Sakkary, Adam Jo J. Elatico, Darwin Gomez, Ricky Nellas, Raymond J. Owens, William Zuercher, Iva Navratilova, Conor R. Caffrey, and Konstantinos Beis, ACS Medicinal Chemistry Letters, 13, 11, 1715-1722 (2022). DOI: https://doi.org/10.1021/acsmedchemlett.2c00248